I don’t know anyone who has been involved with Tobacco NETs that hasn’t said, “I sure wish there was a way to make these extractions go faster!”
Well, to explore how to do that first we have to look at the plant cell structure that holds the flavor we are trying to get at then we have to look at the chemistry of solubility.
This post is in two parts, first part is a discussion of cell wall chemistry and solvents. The second part is my version of “HowTo Do It”. If the chemistry is not of interest to you just jump to the “How To” part.
Chemistry: Solubility and Cell Walls:
We must solve two problems:
1.) How to get inside a cleverly designed ‘lock box’ ?
2.) Once we open the lock box how to ’steal’ the items inside ?
How to get inside a cleverly designed ‘lock box’ ?
Locked away inside a rigid box that Mother Nature made called a cell wall is the important flavor components that we want to extract. For brevity from now on I will just refer to them as “flavonoids”, but in fact we are a talking about a broad range of amino acids, polyphenols, aromatic acids, Lignin, and other phenolics. There are sugar esters, beta-o-glucopyranose (a glucose tetraester) and one of the most important chemicals believed to carry much of what we call the ‘essence’ of tobacco flavors is the sucrose tetraestes
A major function of a plant cell wall is to withstand the osmotic turgor pressure of the cell, so the cell wall is built for lateral strength.
Arranged into layers of cellulose microfibers embedded in a matrix of pectin and hemicellulose, the cell wall is at least 0.2 μm thick and completely coats the outside of the plant. The porosity of the matrix permits soluble factors to diffuse across the cell. However, the cell wall is a selective filter that is more impermeable than the matrices surrounding animal cells. So what we have here is a complex, insoluble polymer of phenolic residues, and lignin which is a strengthening material in all cell walls.
This is not a trivial ‘lock box’ that Mother nature invented. Let’s look at a diagram:
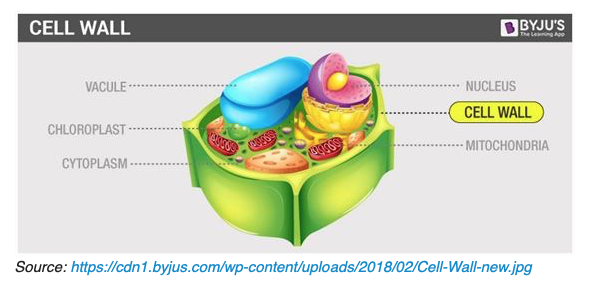
So how to we break open the lock box? We know that if we just put the tobacco leaf sample in a jar and put it on the shelf at room temperature, we will have to wait a long time for the solvent to diffuse into and out of the cell wall and while it is there, we hope it dissolves enough “flavonoids” to do us some good. It is obvious that if we can ‘do something’ to that cell wall we might have better luck. Many NET experimenters have discovered that if we use a procedure usually referred to as a “hot bump” , that is, during the first 6-12 hours of the maceration raise the solvent temperature to 115F-165F we can take months off the extraction times. What is happening when doing that? And what can we do to improve the hot bump?
It is known that via osmosis, of water into the vacuole (see the diagram above) the vacuole expands and generates a hydrostatic pressure, called turgor, that presses the cell membrane against the cell wall. Turgor is the cause of rigidity in living plant tissue. In the mature plant cell, as much as 90 percent of cell volume may be taken up by a single vacuole; immature cells typically contain several smaller vacuoles. It is entirely possible that during the “hot bump” this mechanical pressure increase is rupturing the cell wall, or at the very least providing ‘fracture lines” that provide channels for solvent transfer in the long many month infusion process. A sharp eyed observer of NETs had this to say:
“The phenomena that I have noted - when early on in a (~125 *F) beginning warming phase of maceration, the leaf-bits seem to become a bit “crispy” (noticed when stirring) for a few hours early on, which then goes away as the leaf-bits appear to “soften” again - (may be) the process of warm PG solvent initially entering the plant-cell “vacuoles”, swelling the cell structure volumes from inside, followed by pressure equalization.”
Doing tobacco NETs for our vaping pleasure we are not the first to come up against the cell wall problem noted above. Substantial industrial interest exists in solving the exact same problems. The preferred industrial solutions involve a brute force approach. They apply mechanical shear forces to simply ‘tear the cell wall apart’. The two major manufacturers of equipment for these purposes are: Hielscher – Ultrasound Technology and Silverson (very sorry but the forum software does not allow URL links, you can google these excellent and informative sites maintained by these two companies)
Let’s see what they have to say about the techniques:
“Ultrasound-assisted extraction is based on repeating high pressure and low pressure cycles. These alternating high pressure and low pressure cycles of 20,000 times per second of sonication create intense shear forces and liquid jets. This extreme stress overcomes the selectivity of membrane, perforates and breaks the cell wall, and results in a high mass transfer between the inner cell and the surrounding solvent.”

The problem of cell wall destruction has come to the attention of other industrial sectors. In waste water management and recycling operation there have been chemical digestion techniques using enzyme and bacteria technology. I feel this is a little outside the practical reality for our kitchen chemistry DIY efforts, so I will not go into depth about those techniques.
However, Ultrasonic is very much in the practical and low cost realm of us DIY types. I have personally used the technique with great success. I will give you details in the How-To Part of this post. Let’s move on to:
Once we open the lock box how to ’steal’ the items inside?
The answer is ‘chemical solubility’. Unfortunately we can’t turn this post into a two year course on chemistry, but very briefly the considerations here involve a little understanding of solvent polarity and things that control partial solubility. In other words, everything is not just simply ’soluble’. So what, in the realm of our “flavonoids’ is soluble, in what solvents, and to what degree?
I think the best way to deal with the very complicated chemistry is to start first by giving you the final “take-away” from all the details. The most important thing to realize, that will help you get better “flavor extractions” is that because there is a broad range of solubility of the components of both natural pure leaf flavonoids as well as many added-in artificial commercial flavoring in many pipe and cigar tobacco samples, it is possible to “balance” or “weight” the final flavor profile by using various solvents and using those solvents in particular ways when acting upon the sample processed. Careful selection of the solvents is essential. So using solvents to extract from tobacco is a very much “goal oriented” project. You must first decide what do I want to do? What is, exactly, the goal? What is the flavor profile I am trying to finally wind up with? From that you can pick and choose various solvents and various methods of application for these solvents that will achieve the goal.
For example, let’s say for this round of extraction you have, not a Pure Leaf sample, but rather you have a heavily cased commercial cigar like something from the ACID line of products. You decide that you really like the “Herb Flavor” rather than the binder or ligero leaf of the cigar and want those herbs and casings to predominate. Then you would choose solvents and application techniques like this:
From the data charts below we note that the actual component of tobacco leaf flavor, a broad range of amino acids, polyphenols, aromatic acids, Lignin, and other phenolics, sugar esters, beta-o-glucopyranose (a glucose tetraester) and sucrose tetraester etc, are either not soluble at all in Ethanol, or in some cases, they are ~slightly~soluble only at elevated temperatures. However we note that the artificially derived casing flavors are very soluble in Ethanol, as this is a result of the chemistry in use at flavoring houses that manufacture them. Their customers usually require oil and ethanol solubility as a specification in the product for follow on reasons in their food manufacturing processes. You would choose Ethyl Alcohol over, PG or Water as the solvent of choice for your goal of “casings over natural tobacco profile”.
Further you would use a “modifier” of the application technique that is very short (12Hr, not multi month) extraction times. Since the cell structure of the leaf wall as designed by mother nature requires destruction of the wall or extended (multi month) infusion times to get at the “tobacco essence” flavor components that are locked deep in the cell, if you “short stroke” the time frames then you balance and weight the extracted soluble elements to what the solvent carrier can most readily physically access, and that is the toppings / casings that are spray applied to the surface of the commercial tobacco.
It is important to understand that in chemically polar compounds that are “like” each other there is an important feature of physical chemistry that needs to be considered: As a way to explain this think of this experiment:
Get a tumbler full of water and one sugar cube. Drop the cube to the bottom of the glass and leave it there, no touch, no stir, just sit and watch it. What happens ? Slowly it dissolves, but in a very particular way: with higher density of dissolved sugar nearer the cube, less and less concentration of sugar as you get farther from the cube. The high density area nearer the cube actually slows the rate of further solubility.
What’s happening there?
What is carrying the dissolved sugar away from the cube is: Two things: one is ‘Brownian motion’ that is how molecules behave in a liquid, they sort of nervously move around and disperse themselves, The other is flows or ‘streams’ in the water driven by convective heat currents. This heat energy comes from localized heating and cooling from uneven heating and cooling on parts of the jar. Think of a lava lamp, that is convective heat driven motion.
All this convective and Brownian motion is occurring very slowly in an ‘impatient human time frame’. If we add a lot more energy to ‘pump’ the carrier liquid continuously over the sugar surface we get enhanced transfer of the sugar into fresh areas of the water that have a low sugar molecule count and then things go MUCH faster. Why is that ?: Because Polar and non polar solvents trying to dissolve ‘like material’ do have a dramatic effect on how completely something can ‘dissolve’, and in some sense, because of this polarity, the carrier liquid “sees” around it and makes a molecular determination about things like polarity of the ‘sugar’ and how much it will allow in near its water molecules. I know this sounds strange but it is a part of the physical chemistry of solubility. If we can ‘drag away’ sugar molecules from very near the water molecule that is a good thing. Particularly if there are polarity issues, and particularly if the carrier will allow only ‘partial’ solubility of a certain substance.
So the ‘ferris wheel’ described below in the HOW TO section of this post is just a way of causing enhanced flow around the tobacco exposing it, more often, to solvent that is in a state that is receptive, because of the issue of polarity, to dissolving more ‘good flavor stuff’ .
Let’s look at some of the components of the “flavonoids” and see how they react to various solubility chemistry:
Here is information of multi solvent solubility using common extraction solvents:
By consulting these data you can make determinations like those above that conclude among other things that, for example, Ethyl Alcohol is less likely to extract full flavor profiles of tobacco, where as Water and Propylene Glycol are more likely.
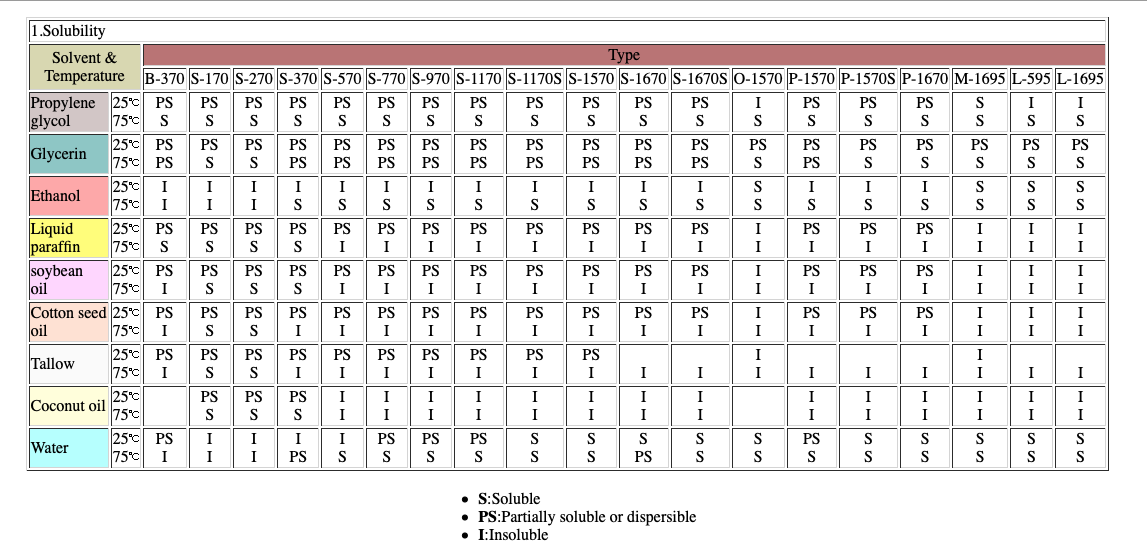
WATER: is called the universal solvent because of its dipolar nature and hydrogen bonding. There are many excellent discussions of water as a solvent in the internet, and just Google “water as a solvent” to read all about it. I am in the process of performing experiments using only water as a solvent for tobacco extraction and am having very good luck with it. I will make a post about that in a month or two. With Water one can obtain very broad spectrum and very “accurate” tobacco flavor profiles. It has the added benefit that it is a very poor solvent of oils and waxes, so the extractions are very ‘clean’ from a ‘coil gunking’ standpoint. I feel water is an excellent choice for tobacco flavor profile extractions, and have been very curious as to why it has not received more attention from experimentally minded NETers. It is of particular interest to PG sensitive persons.
Phenols:
7th Jul, 2013
Debora Fontanini
Università di Pisa
A mixture of methanol/acetone/water will release most of the soluble phenols with subsequent partitioning in ether/ethylacetate. But, depending on the sample you’re working with (we work on cereal flours), you wll need more aggressive strategies to release the bound and conjugate fractions. Hydrolysis with NaOH is recommended prior to partitioning.
Amino Acids:
FROM:
1970
The Solubility of Amino Acids in Various Solvent Systems
Thomas E. Needham
University of Rhode Island
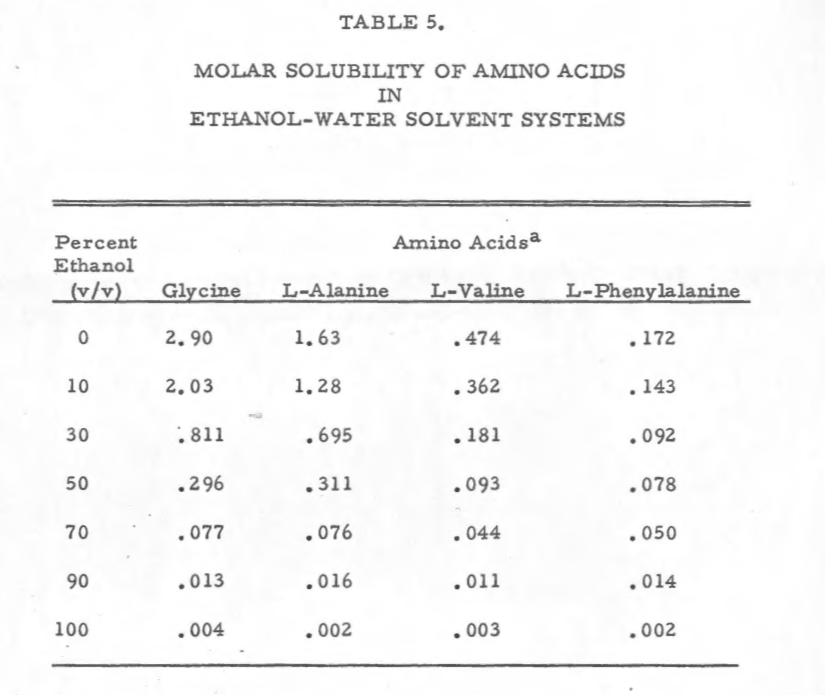
much more good information available from this paper:
https://digitalcommons.uri.edu/cgi/view ... xt=oa_diss
HowTo Do It
I have two methods of speeding up the process.
a.) Ultrasonic water bath table top units.
b.) The Ferris Wheel: This uses agitation to refresh the exposure of fresh solvent to the cells.
(see the Sugar Cube explanation above for why the Ferris Wheel works)
Here is a brief description of each unit:
For around $70 you can obtain units like this:

All these home units have to be re-set every 30 minutes. I considered rewiring to constant ON then using a programmable timer to control the cycles, but thought better of that. For safety I never use the units unless I am around to supervise operation. You do not have to be EXACTLY there every 30 mins to re-set, a lot of ‘slop’ is OK. It is a casual thing.
Not sure if all the China product use the same transducers or not. many brand names on Amazon, not sure if all equal quality or not ?? My procedure is to use a 30 minute ON cycle, then let the Unit and the Extraction REST for 30 minutes, then repeat the cycle. How long to run this cyclic operation is experimental, but for example, in a typical PG Solvent Pure Leaf Virginia sample I will run it for 10 hours. In a 100% Water Solvent Extraction of the same leaf I run it for a total of 24hrs cycle time.
When shopping for a unit, be sure to check that the transducer is at least 60 watts and that it is a tuned horn fee type, not a simple piezo driver that is just simply affixed to the bottom of the water tray. UltraSonic units designed to focus energy for uses like this are very carefully tuned horn feed devices, so there is some risk in dealing with low cost Chins Suppliers that have been known to cut corners. If you get the unit from Amazon, then test upon arrival thusly: just fill it with 2” water, turn it on and observe the portion at the center of the tub. You should see strong convention current flow from that point from a circular area of a diameter of at least 2.5” width. With 2” of water depth in the tub a real focused 60w unit will force much vaporized visible water vapor from the surface. If you put your fingers in the water bath you should feel a very strong vibration that becomes uncomfortable after only a few moments. If you have any suspicion that the unit is not functioning to that level of performance, then return it. I have an Amazon unit from China sold under the iSonic brand and have had very good luck with it, but of course, it is a bit of a gamble and a grab bag with those types of products.
Extractions are placed in Kerr glass jars then you stand them up in the Cleaner. Of course there is a power loss through the glass, but actually the energy transfers very well, and I do not consider it an issue. SEAL THE JAR LIDS DOWN TIGHT !!! You have to do it that way to seal off the extraction from the chamber environment inside the cleaner, because of the heater and the ultrasonic transducer they are generating a very high relative humidity at the surface of the water level. Can’t have that in the extraction jar !
If you use 8oz (1/2Pint) Mason jars, it will fit 4ea at once, so very efficient space wise, but the energy is cut by proportion across those 4 jars, so max ultrasonic energy and effect is to use 1 jar per session.
In short 24 hour infusion cycle Water extractions and overnight Hot Ethanol extractions, where it is intended to not use extended follow-on soak / infusions, I think the method is very beneficial. I feel also that it cuts months off longer term PG type infusions, and the resultant extracts are very broad spectrum in flavor profile.
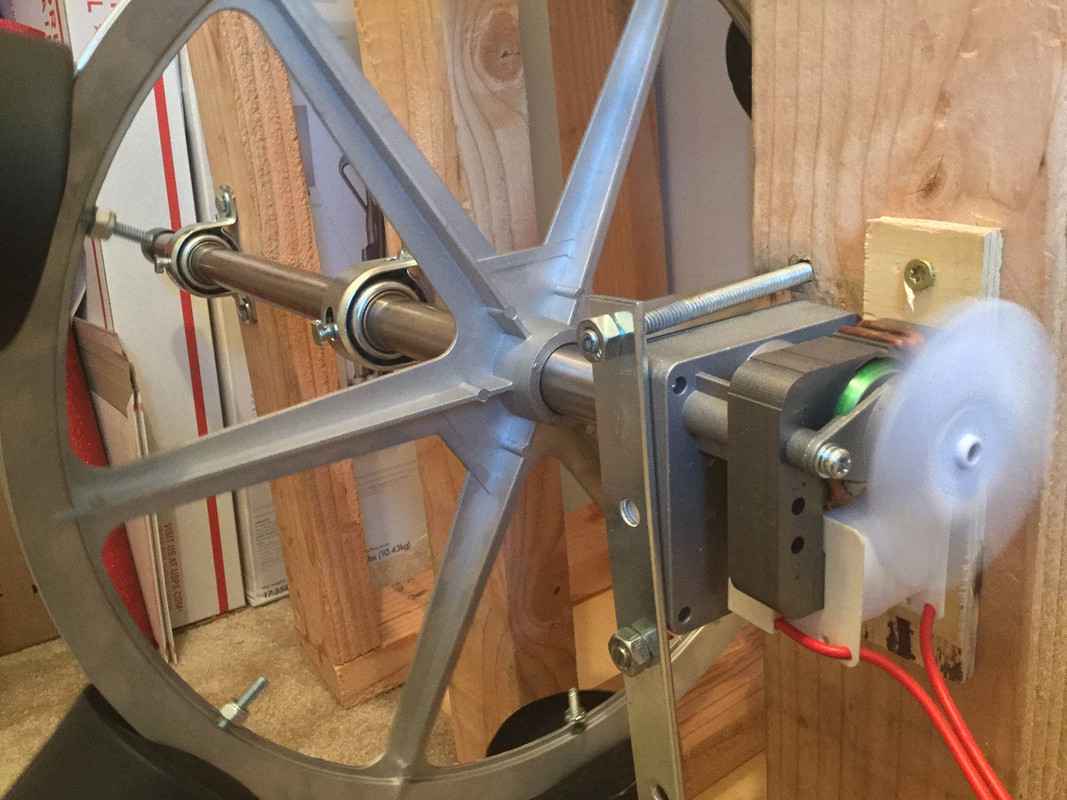
The Ferris Wheel
This is a home brew DIY unit that keeps the solvent “flowing” around the tobacco leaf. The design goals here are that the “pumping” of energy into the solvent is free of typical artifacts of such systems that aerate or infuse oxygen in the solvent. Also the use of the black “beer cozys” that hold the jars effectively shields them from room light. However, I operate the unit in a small closet with the door closed to keep the environment at a steady temperature, and completely dark. The motor is a 1.6RPM shaded pole design that is intended for BBQ and Pellet Stove use. I anticipate it will run trouble free for years. I made the wood frame with an extra slot for an additional wheel, if, in the future, I should decide to scale up the operation to an additional 6 jars.