In many methods of performing NET Extractions there is a required step that involves reducing the volume of solvent after the initial infusion or extraction step.
The purpose of this reduction is to bring the flavor elements to a higher percentage in the solution. This is desirable because at higher percentages of flavor elements the process of using the extract when creating mixable and usable recipes for vaping becomes more manageable, as it gives the recipe creator more latitude to adjust the balance of other elements in the mix, for example the ratios of Propylene Glycol and Vegetable Glycerin
In order for this reduction to work there are a number of physical characteristics of the chemistry of the solvents that must be considered. Some extraction solvents are well suited to reduction operation, some not.
Solvents with the highest vapor pressure and the lowest boiling points are easiest to reduce. Of the common extraction solvents: Ethyl Alcohol is at the top of the list of the most easily reduced solvents. Water, although not a commonly used solvent is somewhat more difficult to reduce, but still very manageable. Propylene Glycol is one of the most commonly used extraction solvents but it is virtually impossible to reduce as its vapor pressure is very low and it boiling point very high at 370F (189C). In the commercial world, far beyond our home kitchen DIY efforts, solvents like Hexane and C02 are common but those solvents are not actually reduced, rather they are completely removed by very expensive and complex commercial processing equipment that is completely out of reach of the average kitchen DIY hobbyist.
So what are techniques within reach of the average DIY mixologist when making NETS?
There are two general categories of solvent reduction. One is low temperature reduction and the other is high temperature reduction. Because the 'flavor element in the NET extract are, or can be, very delicate molecules that have adverse reaction to higher temperatures, the lowest possible reduction temperatures are necessary. My experience is mostly with Tobacco extraction. Over many observations and experiments, I have determined that temperatures above 115F should be avoided at all stages in the NET Tobacco extraction process.
Low Temperature Reduction:

The lowest temperature solvent reduction technique is vacuum dehydration. This is a common technique in the chemistry lab, and many lower end units, usually made by various Chinese manufacturers, exist and can be used by the home DIYer.
PRO: of vacuum dehydration is that the lower temperatures do not degrade the sample as a result of heat related impact to the delicate flavor molecules.
PRO: is that with properly designed (but very expensive) equipment the solvent can be recovered and later reused
PRO: to vacuum systems is that they can be very fast to perform extractions with ~100mls of solvent extracted in matter of minutes.
PRO: Since the system is sealed there is minimal risk of sample contamination with dust, bacteria and foreign material from air borne sources.
PRO: Vacuum pumps that can reduce the pressure to the necessary point can be had on Amazon for $50 to $60. And vacuum Chambers to hold the sample as well as the necessary gauges and hoses can be had as “kits” for another $60.
CON: Like my discussion elsewhere on this forum regarding use of centrifuges where I point out that “this is not for everyone, know your limitations” This also applies to vacuum dehydration, as the systems can become very complex in a hurry and quickly swamp what is supposed to be a casual fun DIY Hobby into a quicksand mire of engineering, maintenance, and endless troubleshooting. For example the phenomenon of so-called “bumping” (a sort of uncontrolled and very messy explosion of the sample) is very common in even high end professional roto-vacs, so the method is anywhere from trouble-free.
CON: The cost and complexity of such a system rises very fast if the system uses ‘moisture traps’ or ‘cold traps’. Although, from a physical chemistry’ standpoint it is not necessary to include these very expensive ‘traps’. However as a practical reality it is necessary because otherwise the solvent vapors will enter the pump chamber and lead to a very much shorter life of the pump.
There are ‘work-a-rounds’ to using traps. One is to change the pump oil after every reduction session. If reducing the the sample by ~100mls of Water or Ethanol, then simply drain and change the pump oil. This has a couple of drawbacks. One is that it’s a hassle dealing with the oil and the change operation itself. The other is that high quality vacuum pump oil is a very pure degassed oil, that is expensive! Typically such oil is ~$50 a gallon, so the on-going cost adds up. Further the oil change work around is only a partially successful way to deal with the issues, and eventually, the pump will fail and must be replaced.
If you decide to engineer a Low Pressure System this following chart may be of some help:
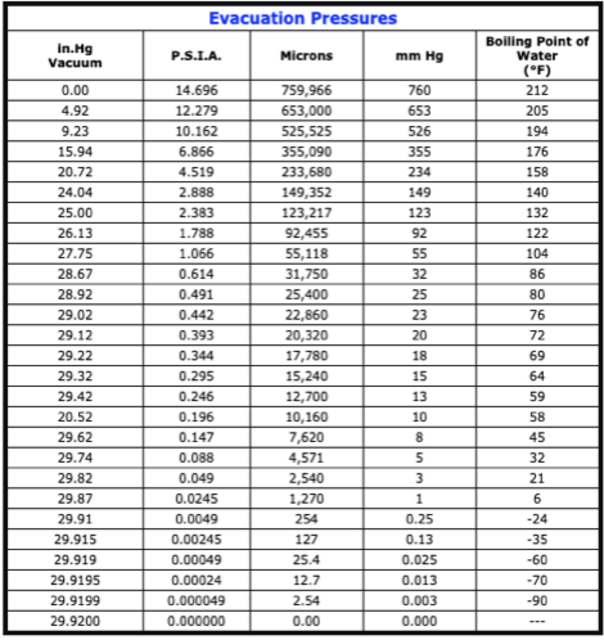
High Temperature Reduction:
I want to emphasize that I am using the term High Temperature in a very specific way. Temperatures above 115F should be avoided at all costs, as serious degradation of the important flavor molecules can occur over 135F. This might be a good place to point out that in the above discussion of vacuum dehydration that process continuously lowers the sample below room temperature and continues to get colder as the process proceeds, So typically samples in a vacuum system can drop 10 to 20 degrees below their starting room temperature. The reason is physics, but we wont go into that right now.
In contrast High Temperature Reduction proceeds well above room temperature. It is critical that the system either have accurate temperature controllers to regulate the heating elements, or that it be cleverly designed to ‘stabilize’ and perform in equilibrium over however many hours that the reduction takes place.
CON: A disadvantage of High Temperature Reduction is that it is a slow technique, with hours required, as compared to the mere minutes required in vacuum systems.
CON:Since the system is NOT sealed there is risk of sample contamination with dust, bacteria and foreign material from air borne sources.
PRO: Since the process of heating up a sample is so straightforward with no exotic lab equipment involved it has the distinct advantage that such methods can be accomplished with little outlay of money. Typically under$20, or even free if you have a good hobby junk box in your back bedroom closet! This is in sharp contrast to vacuum systems that can climb to hundreds or even thousands of dollars in equipment cost.
PRO: The systems are simple and trouble free to operate over long periods, and if properly designed and assembled carefully require little maintenance.
Many rigs have been created to accomplish the task of High Temperature Reduction. If you have one that you like, please post it as a response to this thread. It is always fun to see what others have created.
Here is my favorite DIY set up:
So here is something that you can put together for under $20 Actually, most people have the parts hanging around in a junk box anyway. I scrounge my local ‘thrift store’ and get this type of stuff for pennies.
The key to this setup is to use a 24volt muffin fan and drive it with a 12volt power pack. This gets you a very desirable reduced airflow. So the only item most people will not have around is the 24v fan, but I will provide a link on Amazon.
The setup stabilizes at about 100F after running for an hour and it holds that temp indefinitely. So I think we can tolerate that as acceptable and it should not aggravate loss or alteration of flavor profile. It will evap off ~100 mls of water in 6 hours.


Amazon.com: uxcell 60mm x 15mm 24V DC Cooling Fan Long Life Sleeve Bearing…

Amazon.com: TMEZON 12 Volt 2A Power Adapter Supply AC to DC 2.1mm X 5.5mm Plug…

Amazon.com: Home-X Mug Warmer, Desktop Heated Coffee & Tea - Candle &…

Amazon.com: Frost King Air Conditioner Filters, 15"x24"x3/16", Open cell foam:…

Amazon.com: New Star Foodservice 42917 Stainless Steel Measuring Spoons and Cups…
